Histogram of yeast cell size (beer)
How can you use the histogram of cell size?
The histogram (or the graph) of cell size displays the distribution of cell sizes in the sample, which typically range between 6 and 10 µm.
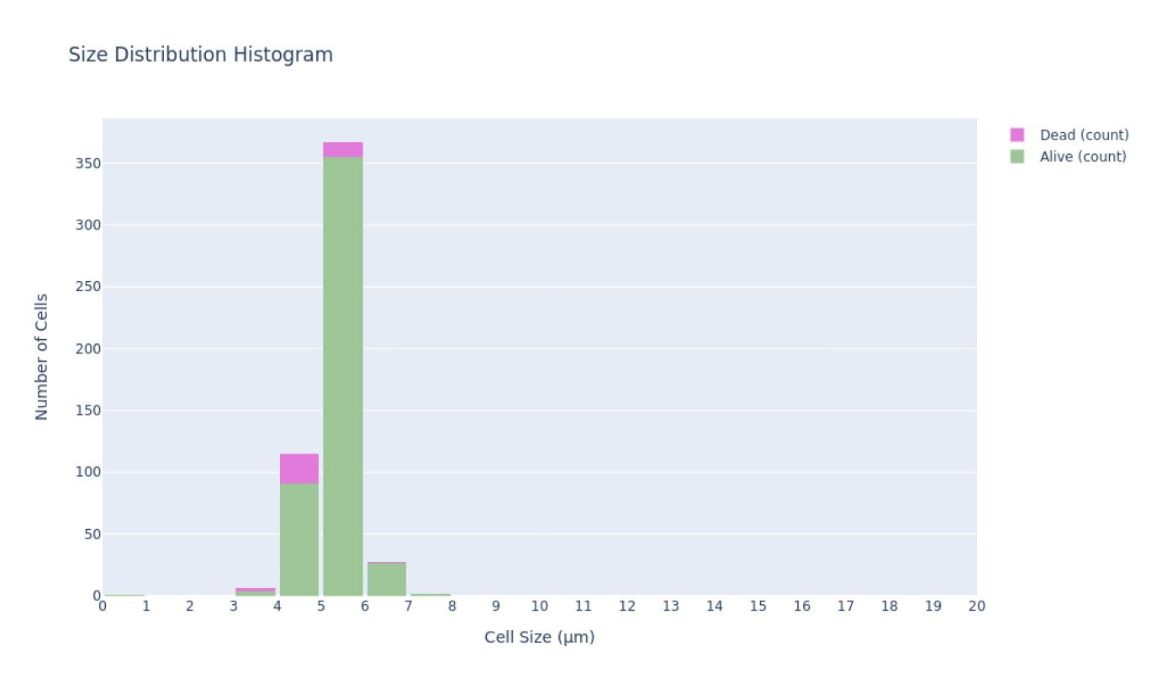
By looking at the histogram along the fermentation process you’ll be able to see how the cells are evolving. Usually, overall larger cells indicate an older yeast population, as the cells grow larger with each budding. As mentioned in the article on the budding index, at the beginning of the fermentation it is to be expected that the cell sizes will be smaller, while by the end of the fermentation single cells will become larger.
Now, yeast cell size has for a long time been intuitively considered a useful marker for predicting the flavor and aroma stability of beer. Still, given that there are so many other very important parameters for brewers to consider (such as yeast cell concentration, yeast viability, the budding index, etc.), but also given that the means to actually measure the size of the yeast cells were only relatively recently fully developed, there has been little attention given to cell size.
However, the implications of a study published in 2003 in FEMS Yeast Research on The impact of brewing yeast cell age on fermentation performance, attenuation and flocculation are telling:
Knowing the distribution of cells by size within the fermentation vessel can help brewers put in place a proper cropping process for “serial repitching.”
Yeast cells increase in size as they age and replicate. And, according to the authors, “the rate at which each cell sediments is believed to vary according to its replicative age. Consequently, sedimentation results in the formation of zones enriched with cells of a particular age. […] Harvesting yeast may therefore select for a population with an imbalance of young or aged individuals, depending on the cropping mechanism employed.” But this begs the question “why would you want to select your yeast by size?” leading us to the second important point.
Younger (and usually smaller) cells perform in a different manner during fermentation than older (and typically larger cells).
According to the authors of the study:
“Cell age influences the rate of sugar utilisation during fermentation”, which can have a serious impact, particularly (but not exclusively) on the lag phase. “Analysis of the initial stages of fermentation”, write the authors, “indicated that virgin cells were slower to begin utilizing sugars when compared to mixed aged cultures. This is indicated by the extended lag phase prior to the maximum rate of sugar utilisation. […] S. cerevisiae cells must meet certain requirements prior to passing START during the cell cycle, one of which is attaining a critical size. Virgin cells (AN: young cells that haven’t replicated yet) do not meet these requirements initially and in order to achieve a specific size they must assimilate nutrients and convert them into biomass, which is time consuming. In addition, it has been demonstrated that the speed at which cells exit from stationary phase is influenced by the severity of the stress period previously encountered. It has been established that virgin cells do not recover from cold shock as rapidly as older individuals.” Furthermore, write the authors, “Virgin cells also fermented at a reduced rate during the middle stages of fermentation compared to non-virgin and mixed aged cultures, although there was no significant difference between the rate of fermentation for non-virgin and mixed age cell cultures.” So while a population of either aged cells or mixed aged (and implicitly size) cultures will ferment at an optimal rate, a population of young, virgin cells is expected to perform at a reduced rate.
As for the importance of these findings, the authors draw attention to the fact that “The increased fermentation time observed for virgin individuals is of particular significance to the brewing industry as an extended fermentation time has a direct impact on plant efficiency, with subsequent financial implications.”
So to sum up all the info so far, assessing the cell size of your yeast population can give you an important indication of how long it will take the yeast to move from dormancy to fermentation and how the yeast will perform along the entire fermentation process. Moreover, by trial and error, you can perfect your cropping process so that you only select the best-performing yeast, i.e. a population of yeast that contains a healthy mixture of different-sized cells. As noted by the authors, while other studies have shown that the older cells accumulate at the bottom of the fermentation vessel, the results of their laboratory tests suggested that the actual location of aged cells within the cone may vary depending on the yeast strain used and the dimensions and shape of the fermentation vessel. Hence the need for a trial-and-error process.
In addition to what has already been said, the authors of the study also note that older (and larger) cells may be more efficient at flocculating.
“Analysis of the flocculation characteristics of each yeast fraction”, they note, “indicated that aged individuals were more efficient at flocculating than younger counterparts, irrespective of their brewing classification (ale/lager).” And this is important because “yeast flocculation provides a natural mechanism for the removal of yeast from beer at the end of fermentation.”
So the distribution of cell size within your yeast population may also give you a good indication of how the yeast will flocculate. Why is this important? you ask again. Because, as the article concludes, “It is suggested that within a full scale fermentation vessel aged individuals displaying an enhanced flocculation potential may sediment early, causing cell numbers in suspension to decrease and reducing the efficiency during the final stages of fermentation in terms of speed and attenuation. Alternatively, selection for a population comprising a large proportion of younger cells during serial repitching may result in slow beer clarification, yeasty off-flavours and filtration issues as a result of weak flocculation.”
Finally, other studies have shown that the yeast cell membrane of larger cells may contain more ergosterol, which increases the ethanol resistance of the yeast cells. Basically, as yeast cells age, the content of ergosterol and unsaturated lipids increases, shielding them from the effects of the increasing concentration of alcohol, as the fermentation advances. This can be particularly important when you’re trying to fix stuck or stalled fermentation by inoculating more yeast. In this case, selecting a population of yeast that is larger in size will increase the chances that the yeast will not be instantly killed off by the ethanol that has already formed in the tank.